
Facility
Situated in the heart of Research Triangle Park in Raleigh, North Carolina, this brand-new, cutting-edge cGMP facility adheres to strict regulatory standards set by the FDA to ensure the safety, efficacy, and quality of all our products.
Azzur Cleanrooms-on-Demand – Raleigh, LLC
1101 Shiloh Glenn Drive, Suite 200A
Morrisville, North Carolina 27560
TomorrowMed Pharma, LLC
1101 Shiloh Glenn Drive, Unit 1108
Morrisville, North Carolina 27560
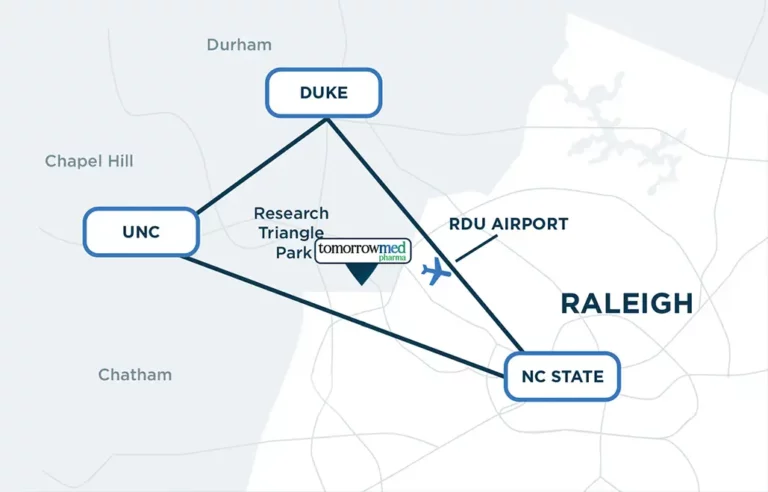

- Located in the heart of RTP, the largest research park in the U.S.
- Located just off I-40 & I-540
- 10 min. from RDU International Airport
- Proximity to top universities, hospitals, & life science campuses

FDA Registration and Compliance:
TomorrowMed Pharma is registering with the FDA and in compliance with Current Good Manufacturing Practices (cGMP). This ensures that all products are consistently produced and controlled according to the highest quality standards.
Enhanced Safety and Quality Control:
We are subject to regular FDA inspections, during which our processes, facilities, and products are rigorously evaluated to ensure compliance with federal regulations. This oversight helps us maintain high levels of safety and quality in the medications we produce.
Assured Sterility and Potency:
Stringent testing for sterility, potency, and stability is a regular part of our practice. This testing ensures that our compounded medications are safe for patient use and maintain their effectiveness throughout their shelf life.
Custom Compounding Services:
We have the ability to create medications tailored to unique patient requirements, such as specific dosage forms, strengths, and combinations that are not commercially available. This customization is particularly important for patients with allergies to certain ingredients or those requiring specific dosages.
Support for Healthcare Providers:
We offer reliable and consistent access to high-quality compounded medications, supporting healthcare providers in delivering optimal care. By partnering with TomorrowMed Pharma, healthcare providers can ensure they have access to the necessary medications without the constraints of traditional pharmacy compounding limitations.


